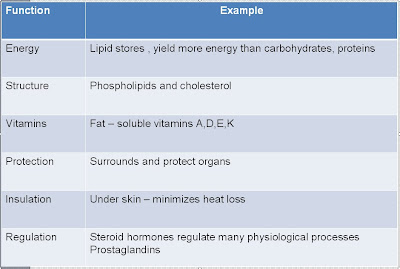
Ø Respiration
Ø Ingestion & Digestion of nutrients
Ø Elimination of wastes
Ø Perform functions
Ø Reproduction
Cytoskeleton provides structure and holds organelles in place
Microtubules provide structure and also transport mechanism for substances from one part of the cell to another.
Proteins of the Cell Membrane
Ø Span entire plasma membrane
Ø Interact with intra and extracellular fluids
Ø Pores for passage of water or other substances
Ø Gates for some ions & facilitated diffusion
Ø Peripheral proteins
Ø Attached only to one surface
Ø Mostly intracellular (G-proteins are a good example)
Ø Attached to intracellular end of integral proteins
Ø Act as enzymes when signaled by integral proteins
Ø Carry message into cell
Proteins of the Cell Membrane
Ø Carbohydrate plus protein
Ø Extracellular
Ø Negative charge to resting cell membrane
Ø Many are hormone receptors (e.g. insulin)
Ø Cell membrane hormone receptors (extracellular)
Ø Glycogen (intracellular)
Ø Energy storage (animal starch)
Ø Insoluble polysaccharide
Ø Liver and skeletal muscle
Ø Modest energy storage compared to fat
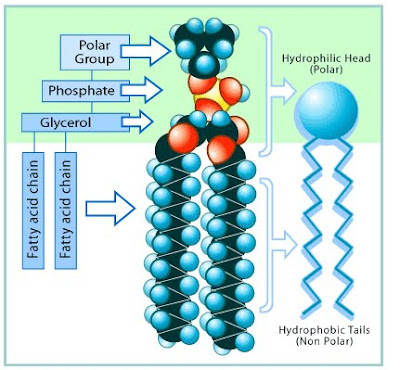
Lipids of the Cell Membrane
(Plasma Membrane)
Ø Cholesterol: in bi-layer. Helps determine degree of permeability of cell membrane to water-soluble substances.
Ø Triglycerides: Energy storage in adipose tissue.
Ø Endoplasmic reticulum
Ø Ribosomes
Ø Golgi apparatus
Ø Lysosomes
Ø Peroxisomes
Ø Mitochondria
Ø Proteasomes
Ø Nucleus
Ø Cytoskeleton
Ø Assemble transfer RNA for packaging into peptides and new proteins
Ø Located in the cytosol and on the rough endoplasmic reticulum.
Ø Interconnected canals and tubules
Ø Digestion of some compounds
Ø Storage of Ca++ ions used in muscle contraction
Ø Synthesizes carbohydrates and Lipids
Ø synthesis of lipids and steroids, metabolism of carbohydrates, regulation of calcium concentration, drug detoxification, attachment of receptors on cell membrane proteins, and steroid metabolism
Endoplasmic Reticulum
Ø Covered with Ribosomes for protein synthesis.
Ø Production of most proteins needed by the cell
Ø Synthesizes phospholipids and cholesterol
Ø Synthesizes the enzymes of glycolysis
Ø Main location for energy production via phosphorylation of ADP to ATP.
Ø Convoluted inner membrane (matrix) Contains components of Citric Acid (Krebs) cycle.
Ø Outer surface of inner membrane contains cytochromes which make up the electron transport system.
Ø Contain DNA & processes for self replication.
Nucleus and Nucleoli
Ø Nucleus
Ø Bilayer nuclear membrane
Ø Largest of the organelles
Ø Contains genetic material (DNA) for cell reproduction)
Ø Contains DNA codes for protein synthesis
Ø Contains nucleolus
Ø Nucleoli (may be multiple nucleoli)
Ø Produces ribosomes for protein synthesis.
Ø Thus, contains the information for the cell functions.
Ø Peroxisomes: Degradation of amino acids, fatty acids and toxic foreign substances. Produce H2O2
Ø Cilia: Movement of extracellular fluid and contents.
Ø Microvilli: Increase surface area for absorption (brush border of small intestine).
Ø Flagella: Locomotion (sperm cell)
Ø Proteasome : Degrades proteins